This paper reflects the research and thoughts of a student at the time the paper was written for a course at Bryn Mawr College. Like other materials on Serendip, it is not intended to be "authoritative" but rather to help others further develop their own explorations. Web links were
active as of the time the paper was posted but are not updated.
Contribute Thoughts |
Search Serendip for Other Papers | Serendip Home Page
|
Biology 202
2000 Third Web
Report
On Serendip
Circadian Rhythms
Andrew B. Hollander
Long ago, before the Great Clock, time was measured by changes in heavenly bodies: the slow sweep of stars across the night sky, the arc of the sun and variation in light, the waxing and waning of the moon, tides, seasons. Time was measured also by heartbeats, the rhythms of drowsiness and sleep, the recurrence of hunger, the menstrual cycles of women, the duration of loneliness. Then, in a small town in Italy, the first mechanical clock was built. People were spellbound. Later they were horrified. Here was a human invention that quantified the passage of time, that laid ruler and compass to the span of desire, that measured out exactly the moments of a life. It was magical, it was unbearable, it was outside natural law. Yet the clock could not be ignored. It would have to be worshipped. (Alan Lightman, Einstein's Dreams. Warner Books, Inc. New York, NY. 1993.)
At first glance, it may seem as if the clock, which human society worships and is slave to, is a human invention. This clock is a way of keeping track of time, assuring that each day is similar in duration and period to the previous, and the next. However, upon closer inspection, biology has proven that the clock is part of every living organism's genetic code and the clock mechanism is controlled by the brain. So, in some sense, the clock is not a human invention, but a product of evolution that controls the processes of life that are vital to the survival of every species and individual. What is this biological clock and how does it work? Why is it important for humans to understand the workings of this natural timepiece?
 The biological clock is scientifically referred to as the circadian rhythm. (Latin: circa means about, and dia means day.) (1) These rhythms show an approximate period length of 24 hours. Perhaps the most powerful evidence that circadian rhythms exist is that daily rhythms, such as sleep and wake, can exist indefinitely in an environment without any time cues. In a 24-hour day with light dark cycles, the animal should maintain a 24-hour period. In situations without the cues, animals exhibit periods a little less or a little more than 24 hours. An actogram of a fly was made that indicated that the fly was active when it was light and inactive when it was dark. When all light was removed, the cycles continued, although each day began 35 minutes later and ended
The biological clock is scientifically referred to as the circadian rhythm. (Latin: circa means about, and dia means day.) (1) These rhythms show an approximate period length of 24 hours. Perhaps the most powerful evidence that circadian rhythms exist is that daily rhythms, such as sleep and wake, can exist indefinitely in an environment without any time cues. In a 24-hour day with light dark cycles, the animal should maintain a 24-hour period. In situations without the cues, animals exhibit periods a little less or a little more than 24 hours. An actogram of a fly was made that indicated that the fly was active when it was light and inactive when it was dark. When all light was removed, the cycles continued, although each day began 35 minutes later and ended  35 minutes later than the previous day (2). The clock relies on zeitgebers, outside influences, to entrain it to a 24-hour period (3).
35 minutes later than the previous day (2). The clock relies on zeitgebers, outside influences, to entrain it to a 24-hour period (3).
Circadian rhythms are believed to include at least three elements: input pathways that transmit environmental signals to the clock, the pacemaker, and output pathways through which the pacemaker regulates the rhythms (4). In mammals, the pacemaker or central oscillator are the suprachiasmic nuclei (SCN), which is a bilateral nucleus in the anterior hypothalmamus above the optic chiasm (5). Evidence suggests that this is the site of the biological clock. The SCN is a cluster of about 10,000 neurons on either side of the midline of the brain and if these nuclei are destroyed, there can be no expression of circadian rhythms(5). In experimental animals with destroyed SCN, "central graphting of neuronal hypothalamic tissue containing suprachiasmic nuclei can restore circadian patterning to the activity-rest cycle." (6) Lesions of the SCN destroy circadian rhythms, but when tissue containing suprachiasmic nuclei are transplanted into the animal, the circadian rhythms are restored. Further evidence that the SCN is the sight of the biological clock is that if neonatal tissue containing SCN is dissociated and held in vitro or in vivo, individual neurons display rhythmic electrical firing (5,6). Thus, the individual cells contain circadian mechanisms, but the cells work together to constitute the central oscillator.
There are several input pathways through which the SCN receives information. One route is from the retina by a monosynaptic pathway called the retinohypothalamic tract (RHT). This derives from specialized photoreceptors that sense light. The RHT connects to the core of the SCN. The detection of light is transmitted via the RHT (7). The second passage is the intergeniculate leaflet (IGL), which receives data directly from the retina, but the route separates from the RHT. The IGL terminates in the geniculo-hypothalamic tract (GHT). The IGL path appears to play an important role in the entrainment, synchronization, by non-photic zeitgebers such as motor activity. Another major input route originates in the raphe nuclei. Other inputs come from cholinergic projections from the basal forebrain and histaminergic projections from the hypothalamus(5).
The SCN has several output routes through which the pacemaker influences the organism's physiology. The suprachiasmic nuclei project to the anterior hypothalamus, the thalamus, the lateral and dorsal medial hypothalamus, the IGL, and lateral septal nucleus (5). Another connection occurs in the "subparaventricular zone of the hypothalamus. From this region, projections arise that innervate the upper thoracic intermediolateral cell column and from there projections extend to the superior cervical ganglion sympathetic neurons which in turn innervate the pineal gland." (5) Thus, the SCN is able to regulate the production of melatonin, which is secreted in the pineal gland. (To learn about the structure of the brain, go to the Whole Brain Atlas)
Melatonin secretion follows a circadian rhythm that is controlled by the SCN. There are 2 types of melatonin receptors in mammals, MEL1a and MEL1b. Mel1a has 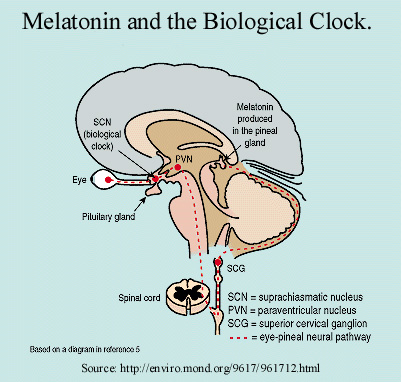 been found in various parts of the brain; however, MEL1b appears only in the retina. MEL1a is believed to mediate circadian and seasonal effects of melatonin while MEL1b affects photoreceptor light sensibility (7). Melatonin levels rise during the night and fall in the morning (5). Injections of Melatonin alter circadian rhythms. Sheep, lizards, birds, and humans have all demonstrated phase shifts due to melatonin. This hormone shifts the phase of the rhythm in a "dark-pulse" direction, opposite the direction of a phase shift resulting from light (5). Exposure to a single pulse of light shortly after the onset of the night cycle, caused delays in the rhythm of mice; pulses delivered later in the night caused an advance in the rhythm (8). Melatonin displays opposite effects, that is, this chemical promoted rhythm advances when administered at night, and caused delays when given in the morning (9).
been found in various parts of the brain; however, MEL1b appears only in the retina. MEL1a is believed to mediate circadian and seasonal effects of melatonin while MEL1b affects photoreceptor light sensibility (7). Melatonin levels rise during the night and fall in the morning (5). Injections of Melatonin alter circadian rhythms. Sheep, lizards, birds, and humans have all demonstrated phase shifts due to melatonin. This hormone shifts the phase of the rhythm in a "dark-pulse" direction, opposite the direction of a phase shift resulting from light (5). Exposure to a single pulse of light shortly after the onset of the night cycle, caused delays in the rhythm of mice; pulses delivered later in the night caused an advance in the rhythm (8). Melatonin displays opposite effects, that is, this chemical promoted rhythm advances when administered at night, and caused delays when given in the morning (9).
There is also a circadian rhythm in histaminergic activity. In an experiment, tests showed that rats had the highest amounts of histamine in the hypothalamus during the dark period, when the rats were most active. Similarly, test on humans have shown the highest levels of histaminergic activity occurred during the day, when the people were most active (10). In rats, the fact that histamine begins to be released before the lights are shut off and histamine neurons increase in activity in anticipation of wakening indicate that histamine is related to a circadian rhythm as opposed to simply being affected by the change in light (10). Unlike melatonin, histamine causes a phase shift similar to shifts caused by light. In experiments done with rats, if histamine was administered in the early dark hours, there was a phase delay. If the histamine was given in the late dark hours, there was a phase advance (10).
The circadian rhythm of Drosophila has been studied and researchers have an idea how the biological clock in the fly works. The circadian rhythm appears to be based on negative feedback loops (11). In the fly, the feedback loop is dependent upon two proteins: PER, which is made by the period gene, and TIM, which is made by the timeless gene. PER and TIM proteins accrue in photoreceptors and in the suprachiasmic nuclei. The circadian rhythm begins around noon when the per and tim genes transcribe the DNA into RNA. This transcription is activated by the heterodimer dCLOCK (dCLK) and CYCLE (CYC) (11). The doubletime gene encodes the protein DBT that regulates the buildup of per and tim RNA (4). Shortly after dark, the PER and TIM proteins associate to form dimmers (4) and enter the nucleus where they repress their own transcription (12). A peak in the PER/TIM complex levels tells the cells to stop transcription. Transcription is terminated about four hours before dawn (4). Before dawn, the PER/TIM complexes disintegrate and per and tim transcriptions begins again by noon.
Scientists have been working on how the PER/TIM complexes break down. It is assumed that the TIM proteins are involved with some other protein that allows for the disintegration. TIM is specifically targeted because it is the only protein that is directly affected by the amount of light. The missing link was identified as cryptochrome (CRY). The molecular nature of how cryptochrome targets TIM is unknown. The effects of CRY on TIM can be explained in two ways. First, CRY and TIM interact in the dark, but only in light does CRY undergo a change that leads to the collapse of the PER/TIM function (12). Another hypothesis is that light allows CRY and TIM to come in contact (12). Experiments have shown that CRY interaction with TIM and the PER/TIM complex is dependent on light. No interaction occurred between CRY and PER, CLK, or CYC(12).
Experiments with fly cryptochrome (dCRY) indicate that dCRY may be the only photoreceptor that influences the fly's circadian rhythm. If an animal is exposed to constant, intense light, the biological clock goes crazy. Mutant flies, called cryb flies, maintain a constant rhythm under intense light (13). The mutant drosophila have a normal clock in dark-light conditions, even though they have a crippled photoreceptor. Researches hypothesize that the cryb still has some functions that were not destroyed. Others suggest that there may be another unknown entrŽe route to the clock (13). There are at least two types of cryptochrome in mammals, Cry1 and Cry2. Experiments with mice with mutated Cry1 or Cry2 or both were used to test the role these genes have on circadian rhythm. Researchers determined that neither Cry1 nor Cry2 was crucial for mouse survival. The activity of mice running on a wheel was used to mark the circadian rhythm. Mice usually start running shortly after dark and continue running for several hours. In normal mice, kept in the dark 24 hours a day, the mice kept a schedule close to 24 hours. Cry1 and Cry2 mutants showed relatively normal activity when kept in a light-dark cycle, but in an all dark cycle, the Cry1 mutants exhibited a cycle shorter than 24 hours and the Cry2 mutants exhibited a cycle somewhat longer than 24 hours (14). Mice that had both Cry1 and Cry2 mutated, their rhythms in a light-dark cycle were fairly normal. However, in constant darkness, these double mutants seemed to have no internal clock; they displayed no periodicity and their running cycle was confused (14).
In the past it was assumed that the same photopigments in the eye were responsible for both vision and input for the circadian rhythm. Opsins located in the retina, which are linked to vitamin A, absorb light and transfer signals to the brain via the optic nerve. The cryptochromes, linked to vitamin B-2, are found in a different part of the retina than opsins, namely the ganglion and nuclear layers (15). However, some blind people are able to maintain a constant biological clock because the part of the retina containing cryptochrome is not destroyed (16). Severing the optic nerve destroys both vision and circadian rhythm (16). Experiments on mice have shown that mice lacking both rods and cones in the retina, and therefore unable to see, were able to maintain a constant rhythm. When the optic nerve was cut, the animal's circadian rhythm was destroyed (15). This observation suggests that there must be some other protein involved than just the opsins, and most believe that this player is cryptochrome (17).
The eyes may not be the only route through which light effects circadian rhythm. Research done at Cornell University Medical College indicates that light directed at the backs of people's knees reset the clock as efficiently as light directed at the eyes (17). To objectively test how the clock was affected, researchers measured body temperature and melatonin concentration in saliva (17). The researchers used a Billiblanket, which is a halogen lamp that directs light through fiberoptic cables inside of a woven pad. The fiberoptic cables separate heat and light, thus the clock would not be altered by heat. Subjects wore these pads on the back of the knee for three hours. Results suggest that light delivered to the back of the knee matched the results of light directed at the eye (17).
Relatively little is known about the working of the circadian system, but the importance of learning about the biological clock is far reaching. By understanding how the clock works and discovering treatments and drugs that can affect the clock can have a major effect on society. The sleep-wake cycle will be understood and thus conditions such as insomnia, delayed sleep phase syndrome, and jet lag will be able to be treated more effectively. Shift workers who suffer from disrupted rhythms and bouts of extreme sleepiness have caused some infamous accidents like Three-Mile Island, Chernobyl, and the Exxon-Valdez oil spill. The circadian clock may influence the cardiovascular system. The morning is the most common time for strokes to occur and blood clots most rapidly in the morning (3). Knowing the appropriate time in the circadian rhythm to administer drugs may increase the effectiveness of the medication while causing fewer side effects. The circadian system affects all organisms' lives and by understanding the working of these complex negative feedback loops, humans may benefit and allow for a healthier, more productive society.
There are still many questions that have yet to be answered before the circadian clock is fully understood. Although evidence so far supports the hypothesized role of cryptochrome, researchers are still not certain that cryptochrome is the missing link in the relationship of light to the clock. Other questions remain about the actual mechanisms by which cryptochrome works and why it is able to cause TIM to break down. The existence of cryptochrome supports the argument of evolution since plants and animals all contain this protein. In plants, CRY seems to be mostly involved with light detection, but in animals CRY seems to have more of a role in the actual working of the biological clock. By studying these rhythms humans may gain more understanding into a common ancestry for all living beings. There is still much research to do before this complex system is understood, but recent advances suggest that the questions being asked and the phenomenon being studied will ultimately lead to the full comprehension of these negative feedback loops.
WWW Sources
1) Your Ticking Clock: Your Circadian Rhythm , A general overview of the importance of the circadian rhythm.
2) Background , A brief introduction to circadian rhythms.
3) Circadian Rhythms: These 24-hour cycles keep you on schedule ,
Points out some of the ways the circadian clock affects health
4) The Molecular Genetics of Circadian Clocks,
A discussion of the genetics involved in the circadian system for many plants and animals
5) Hormonal and Pharmacological Manipulation of the Circadian Clock: Recent Developments and Future Stategies ,
A detailed presentation by Gary Richardson and Barbara Tate about the circadian clock and how it might be manipulated through drugs
6) The Brain, circadian rhythms, and clock genes ,
A paper by Michael Hastings about the clock and molecules that affect the clock and the entrainment to light
7) Rhythmic Signal Transduction Abnormalities: Can Melatonin Bring Back the Groove? ,
A description of how the circadian system affects melatonin secretion. Written by Mike Hanson.
8) Rapid Resetting of the Mammalian Circadian Clock ,
Jonathan Best, Elizabeth Maywood, Karen Smith, and Michael Hastings discuss the circadian clock in this article in The Journal of Neuroscience
9) The miracle of melatonin: fact, fancy, and future ,
An article by Debra Jean Skene about the relationship between melatonin and the biological clock
10) Modifying Effects of Histamine on Circadian Rhythms and Neuronal Excitability ,
A discussion about the relationship between circadian rhythms and histaminergic activity
11) Time Flies for Drosophila ,
A discussion by Audra Scully and Steve Kay about the genetics of Drosophila circadian rhythm
12) Light-Dependent Sequestration of TIMELESS by CRYPTOCHROME ,
A study by M. Ceriani, Thomas K. Darlington, David Staknis, Paloma Mas, Allegra A. Petti, Charles J. Weitz, Steve A. Kay in Science Magazine about how cryptochrome may block the function of PER/TIM complexes in circadian clocks
13) Researchers Identify Unique Circadian Rhythm Photoreceptor ,
A news article announcing the identification of cryptochrome
14) Crying Over Circadian Rhythms ,
An article by Peter Follette about experiments involving Cry1 and Cry2
15) Cryptochromes - bringing the blues to circadian rhythms ,
A study by Paul F. Devlin and Steve A. Kay about cryptochromes and the circadian clock
16) Major Discovery: Scientists Find Eye Pigment Controls Circadian Rhythm ,
An article about the discovery of cryptochromes
17) Biological clocks sense light in obscure ways , A report by John Travis on a study that found light shown on the backs of the knees has the same effect on the circadian clock as light shown in the eyes
on the Serendip web site
 The biological clock is scientifically referred to as the circadian rhythm. (Latin: circa means about, and dia means day.) (1) These rhythms show an approximate period length of 24 hours. Perhaps the most powerful evidence that circadian rhythms exist is that daily rhythms, such as sleep and wake, can exist indefinitely in an environment without any time cues. In a 24-hour day with light dark cycles, the animal should maintain a 24-hour period. In situations without the cues, animals exhibit periods a little less or a little more than 24 hours. An actogram of a fly was made that indicated that the fly was active when it was light and inactive when it was dark. When all light was removed, the cycles continued, although each day began 35 minutes later and ended
The biological clock is scientifically referred to as the circadian rhythm. (Latin: circa means about, and dia means day.) (1) These rhythms show an approximate period length of 24 hours. Perhaps the most powerful evidence that circadian rhythms exist is that daily rhythms, such as sleep and wake, can exist indefinitely in an environment without any time cues. In a 24-hour day with light dark cycles, the animal should maintain a 24-hour period. In situations without the cues, animals exhibit periods a little less or a little more than 24 hours. An actogram of a fly was made that indicated that the fly was active when it was light and inactive when it was dark. When all light was removed, the cycles continued, although each day began 35 minutes later and ended  35 minutes later than the previous day (2). The clock relies on zeitgebers, outside influences, to entrain it to a 24-hour period (3).
35 minutes later than the previous day (2). The clock relies on zeitgebers, outside influences, to entrain it to a 24-hour period (3).  been found in various parts of the brain; however, MEL1b appears only in the retina. MEL1a is believed to mediate circadian and seasonal effects of melatonin while MEL1b affects photoreceptor light sensibility
been found in various parts of the brain; however, MEL1b appears only in the retina. MEL1a is believed to mediate circadian and seasonal effects of melatonin while MEL1b affects photoreceptor light sensibility