Serendip is an independent site partnering with faculty at multiple colleges and universities around the world. Happy exploring!
Cell Membrane Structure and Function
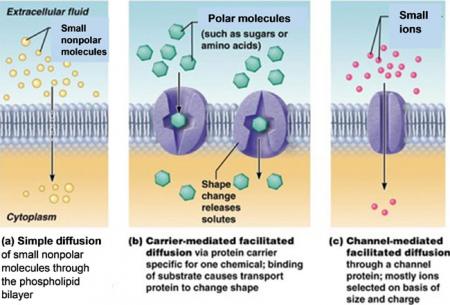
This activity includes two hands-on experiments and numerous analysis and discussion questions to help students understand how the characteristics and organization of the molecules in the cell membrane result in the selective permeability of the cell membrane.
In the hands-on experiments, students first evaluate the selective permeability of a synthetic membrane and then observe how a layer of oil can be a barrier to diffusion of an aqueous solution.
Students answer analysis and discussion questions to learn how the phospholipid bilayer and membrane proteins play key roles in the cell membrane function of regulating what gets into and out of the cell. Topics covered include ions, polar and nonpolar molecules; simple diffusion through the phospholipid bilayer; facilitated diffusion through membrane proteins; and active transport by membrane proteins.
An optional additional page introduces exocytosis and endocytosis.
Download Student Handout: PDF format or Word format
Download Teacher Preparation Notes: PDF format or Word format
The Teacher Preparation Notes provide instructional suggestions and background information and explain how this activity is aligned with the Next Generation Science Standards.
We invite comments on this Hands-On Activity and the accompanying Teacher Preparation Notes, including suggestions for other teachers who are planning to use the activity, useful preparatory or follow-up activities, additional resources or any questions you have related to the activity, or a brief description of any problem you might have encountered. If you would prefer to send your comments or questions in a private message, please write Ingrid Waldron at iwaldron@sas.upenn.edu.








Comments
2020 revision
We have revised the Student Handout to improve student understanding of the important concepts of how phospholipids and proteins work together to accomplish the functions of the cell membrane.
Ingrid
2020 updated version
Where is the updated version with endocytosis and exocytosis?
I see the teacher prep version, but not the student version.
Exocytosis and Endocytosis Addition
I have revised the Teacher Preparation Notes to clarify that page 12 of the Teacher Preparation Notes should be added as page 10 of the Student Handout in order to include exocytosis and endocytosis in this activity.
Ingrid
Is there an image of the
Is there an image of the procedure. I am confused on the bubble right on the surface scenario.
dye on top of water or oil layered over water
The idea of the Procedure (part 2) on page 5 of the Student Handout is to put a very small amount of dye on top of water or oil layered over water and observe what happens. This figure shows the setup.
I hope that helps.
Ingrid
2018 revision
The Student Handout has been entirely revised, with expanded explanations and questions concerning the molecular structure of cell membranes and how phospholipids and proteins combine to form the selectively permeable cell membrane. We have added a second experiment which demonstrates how a layer composed of nonpolar molecules can form a barrier to the diffusion of ions. Also, the Student Handout now includes a brief introduction to endocytosis and exocytosis.
Student handout
Can you give me a possible answers of the student handouts because our class is debating of our experiment's results.
Answers
Please see the Teacher Preparation Notes to find instructions about how to get an answer key.
Ingrid Waldron
Movement/decomposition of IKI
When the bag of starch solution is placed in the beaker of water/IKI, we are wondering if:
1) the IKI moves in AND out of the bag?
2) the water moves in AND out of the bag?
2) if the beaker is left out for several hours (or overnight), the bag becomes very dark whereas the solution in the beaker becomes clear. Is this because all the IKI diffuses in or because of photo-decomposition?
Thank you
Interpreting Experimental Results
Thanks for your questions. Any molecule or ion that can cross the dialysis tubing in one direction can also cross in the other direction. We have observed that, over time, the solution in the beaker becomes much lighter as more of the iodine diffuses into the bag. In our experience the solution does not become entirely clear. Iodine-iodide (KI) solution is less stable in light, but I wouldn’t expect it to break down that rapidly (http://www.onboces.org/safety/msds/S/Scholar%20Chemical/Iodine-Iodide_KI_Solution_355.00.pdf).
Ingrid
2015 revision
The Student Handout has been streamlined for maximum efficiency of student learning and understanding the most important points. The Teacher Preparation Notes have also been updated.
2014 revisions
The revised Student Handout includes clarified instructions for the experimental procedure and improved explanations and questions concerning membrane structure, osmosis, and the use of models to improve understanding of transport across cell membranes. The revised Teacher Preparation Notes clarify the instructions for the experimental procedure.
2011 revision
The Student Handout has been streamlined and the questions have been focused on the topic of diffusion across a selectively permeable membrane.
2009 revision
The major change is the addition to the Teacher Preparation Notes of two ways to modify the activity: (1) to focus on osmosis specifically or (2) to help students develop a more sophisticated understanding of how molecules move across biological membranes.
Post new comment